Iitian faculty explains the above concept in entertaining and conceptual manner.
Allyl and vinyl halides.
Aryl halides or haloarenes in aryl halides the halogen atom is directly bonded to an sp2hybridised carbon of benzene.
Vinyl chloride h 2c ch cl is an example.
In vinyl halides the halogen atom is bonded to an sp2hybridised carbon atom of c c double bond.
In addition the carbon halogen bond is shorter and therefore stronger in aryl halides than in alkyl halides.
The key difference between these two structural components is the number of carbon and hydrogen atoms.
For 3º halides a very slow s n 2 substitution or if the nucleophile is moderately basic e2 elimination.
An allylic halide is an alkyl halide in whose molecule there are one or more halogen atoms on allylic carbons.
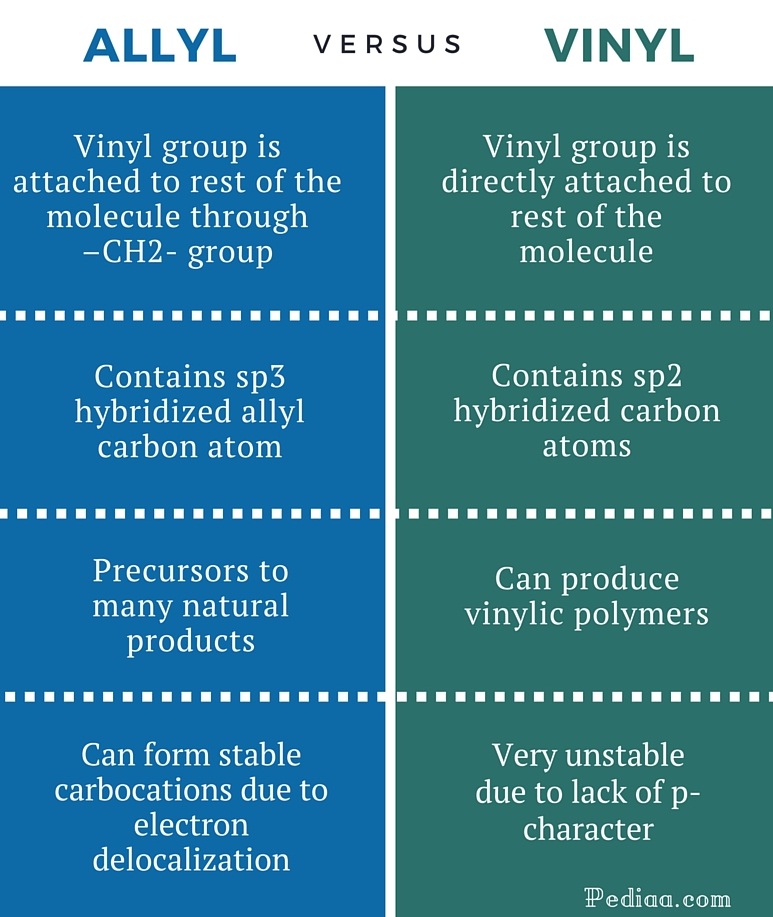
Both groups own a double bond between two carbon atoms where all the other atoms are bonded through single bonds.
Rapid s n 2 substitution for 1º.
It consists of a methylene bridge ch 2 attached to a vinyl group ch ch 2.
Allyl group holds three carbon atoms and five hydrogen atoms on the other hand vinyl group has two carbon atoms and three hydrogen atoms.
An aryl halide has general formula c6h 5x in which an halide group x has substituted the aryl ring.
Likewise phenyl cations are unstable thus making s n 1 reactions impossible.
The general molecular formula for allyl is rch 2 ch ch 2 whereas the general molecular formula for vinyl is rch ch 2.
The carbon halogen bond is shortened in aryl halides for two.
Steric hindrance caused by the benzene ring of the aryl halide prevents s n 2 reactions.
Allyl h 2 c chch 2 rapid s n 2 substitution for 1º and 2º halides.
Polyvinyl fluoride is another commercial product.
Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation.
In high dielectric ionizing solvents such as water dimethyl sulfoxide acetonitrile s n 1 and e1 products may be observed.
For this reason alkenyl halides with the formula rch chx are sometimes called vinyl halides.